Enterprise Application for Rare Disease Data Management & Patient Monitoring
-
Client name
Genetic diagnostics
company -
Industry
Healthcare
-
Location
Germany
-
Duration
2018-2020
Our client is a German biotech company specializing in research and diagnostics of rare hereditary diseases. Our client has many strategic goals but their key purpose is to save precious patient time, address anxiety, and speed up breakthroughs for families battling rare diseases.
Challenges
The company required a secure, scalable enterprise system for their specialists to manage patient data, monitor disease progression, and improve communication. Essentially, the client wanted to introduce a centralized data platform for:
-
Secure patient data collection and monitoring for rare diseases
-
Improving communication between patients and their medical team
-
Efficient control of complex access rights for administrative staff
-
Management of multilingual medical surveys (Quality of Life)
-
Generating deep analytics and reporting on key metrics
-
Ensuring compliance with high security standards for medical data
Solutions
Anadea developed an enterprise web and mobile application that brought our client a centralized administrative platform with three core modules:
- Access management,
- Content management (QoL questionnaires),
- Analytics and reporting.
PHASE 1
Discovery & Architecture Design
-
Requirements Analysis
We mapped workflows for patient data monitoring, role hierarchies, and multilingual survey (QoL) management.
-
Security-First Architecture
Designed an RBAC model with permission inheritance (Write > Read) and Active Directory/LDAP integration.
-
Compliance Blueprint
Defined a documentation strategy for non-medical device certification.
PHASE 2
Core Module Development
-
Access Control Engine
- Built permission groups with Read/Write granularity
- Implemented role templates (e.g., "Reporting" = analytics-only access)
- Developed column-level data visibility rules
-
Survey Management System
- Created dynamic editor for QoL questions/answers
- Engineered translation pipeline with file-based uploads (CSV), a version control with active/inactive states, and a preview mode for validation
- Automated CSV survey exports
PHASE 3
Analytics & Integration Layer
-
Data Visualization Hub
- Developed interactive dashboards with filters (time/patient groups)
- Charting libraries for KPI tracking
- QoL completion rates (Mood/Health/Pain)
PHASE 4
Security & Deployment
-
Entity-Level Permissions
Custom rules for sensitive data (biomarkers, patient records).
-
Authentication
Active directory single sign-on (SSO).
-
Agile Documentation
UML diagrams (BPMN/sequence) + acceptance criteria.
-
Scalability Testing
Validated permission expansion without performance loss.
Key Technical Outcomes
-
1
Single interface for access control, surveys, analytics, and alerts.
-
2
Non‑technical staff can manage multilingual surveys via CSV.
-
3
Automated permission audits and activity logs.
-
4
Exportable analytics for therapy effectiveness tracking.
Core Platform Features
-
Access Control (RBAC)
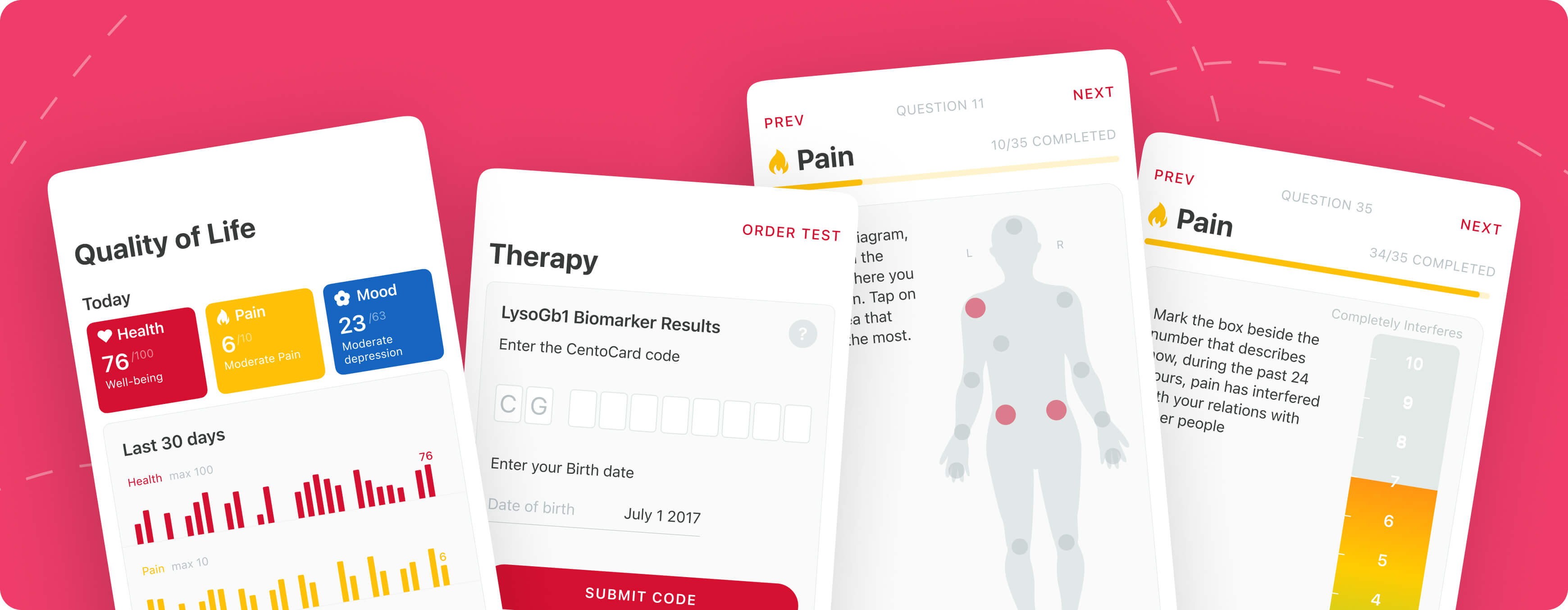
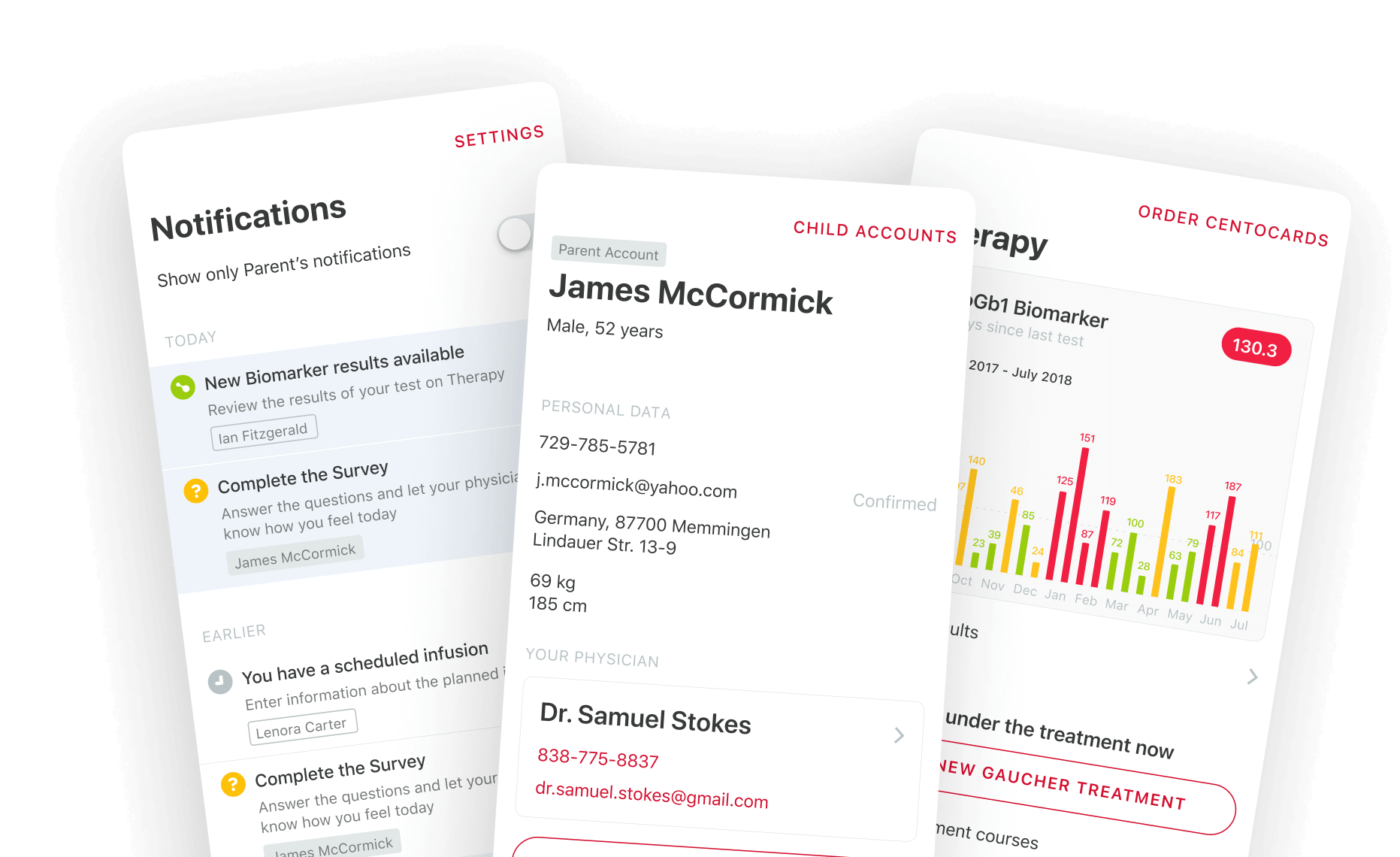
The system controls who sees what. Teams get custom access levels—some can only view basic details, others can edit medical records. This keeps patient genetic data safe while letting doctors focus on treatment.
-
QoL Questionnaire Management
This feature helps medical staff quickly update health surveys for different diseases and languages. It can show how patients feel through pain/mood tracking. Export to CSV is also possible.
-
Analytics & Reporting
The platform turns data into actionable insights. It allows seeing registration patterns, identifying why patients miss appointments, or tracking how pain levels change over time. Visually rich charts and reports help clinics adjust care plans faster.
-
Notification System
It sends automatic alerts for urgent needs: a new patient waiting for assignment, an uncompleted task risking delays, or a lab kit shipment requiring tracking. Nothing slips through.
Need HIPAA-Compliant Patient Analytics?
Services
-
Custom Web & Mobile Application Development
-
Business Analysis
-
UX/UI Design
-
Quality Assurance
Dedicated Team
-
2
Web developers
-
1
Mobile developer
-
1
Business analyst
-
1
PM
Tech Stack

Nexmo

GCM
Firebase
Business Value
-
Enhanced Security & Compliance
RBAC and audit-ready documentation slashed compliance meetings and proved non-medical device status in record time. Researchers focused on cures, not paperwork.
-
Operational Efficiency
The transition from fragmented processes to a centralized administration panel transformed user, permission, and patient data management. Simplified user database maintenance and elimination of duplicate records helped consolidate critical workflows into a single interface.
-
Data-Driven Insights
Raw data found its voice. Dynamic dashboards uncovered patient activity rhythms, quality-of-life shifts, and cohort-specific patterns. These data-driven perspectives empowered the team to adapt research methodologies.
-
Proactive Monitoring
Automated notifications now track critical triggers—high-risk behaviors, decline of system activity, urgent milestones—ensuring no patient signal goes unheard. Real-time vigilance turned reactivity into proactive care.
-
Future-Proof Flexibility
Scalability was woven into the platform’s DNA. New features, user roles, and access scenarios integrate seamlessly without compromising security or system stability.
Contact us
Let's explore how our expertise can help you achieve your goals! Drop us a line, and we'll get back to you shortly.